The ‘oid’ in terpenoids, flavonoids, and cannabinoids has deliberate meaning that imparts specific scientific information about the phytochemical in question. Learning what this ‘oid’ ending means can help to automatically determine several details about a molecule. In most cases, flavonoids and cannabinoids will automatically end in ‘oid,’ but the terpene family of hydrocarbons often don’t and have clear distinctions between terpenes and terpenoids. The details below will explain not only why this distinction occurs, but also how to use this information to make determinations about a particular constituent in cannabis.
‘Oid’ denotes an oxygen atom
The ‘oid’ designation can be quite simple; this ending often denotes the occurrence of one or more oxygen atoms in a particular molecule. In other words, ‘oid’ tells us that a molecule has been oxygenated, and the number of oxygen atoms tells us the degree of this oxygenation. For instance, consider the molecules below by chemical formula:
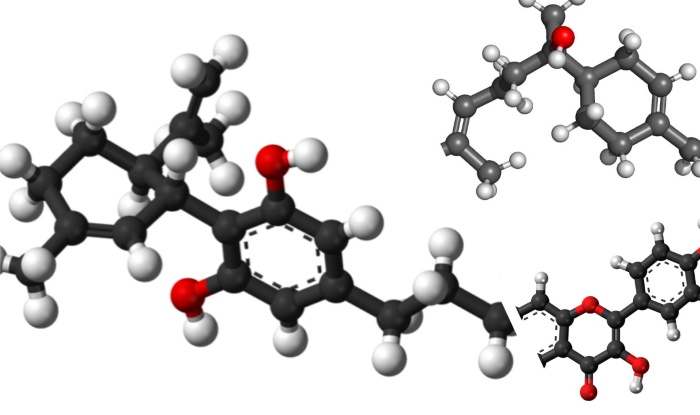
CANNABINOID: CBD (cannabidiol) – C21H30O2
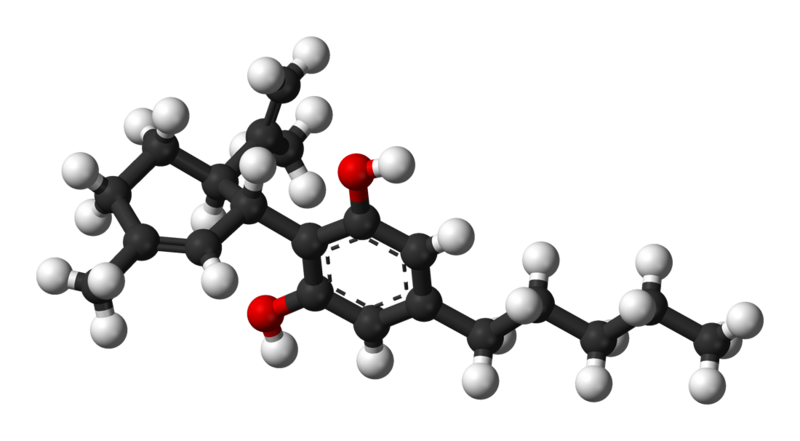
FLAVONOID: Orientin – C21H20O11
TERPENOID: Bisabolol – C15H26O
In each of these chemical formulas, the ‘O’ denotes the occurrence of one or more oxygen atoms, whereas the ‘C’ denotes the number of carbon atoms, and the ‘H’ the number of hydrogen atoms. In the examples above, CBD has 2 oxygen atoms, orientin has 11, and bisabolol has just 1. If we examine the CBD molecule, we see:
The red atoms here are the oxygen atoms present in the CBD molecule, warranting the ‘oid’ designation in this cannabinoid. If we were to examine the precursor molecule to CBD, CBDA or cannabidiolic acid (C22H30O4), we would see 2 additional (red) oxygen atoms.
In the orientin molecule, we see:
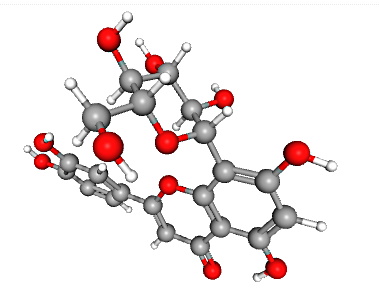
Again, the red atoms here are oxygen atoms, warranting the ‘oid’ designation in this flavonoid. In this flavonoid molecule, there are 11 oxygen atoms, with 21 carbon and 20 hydrogen atoms, hence the C21H20O11 designation.
Finally, if we examine the bisabolol molecule, we see:
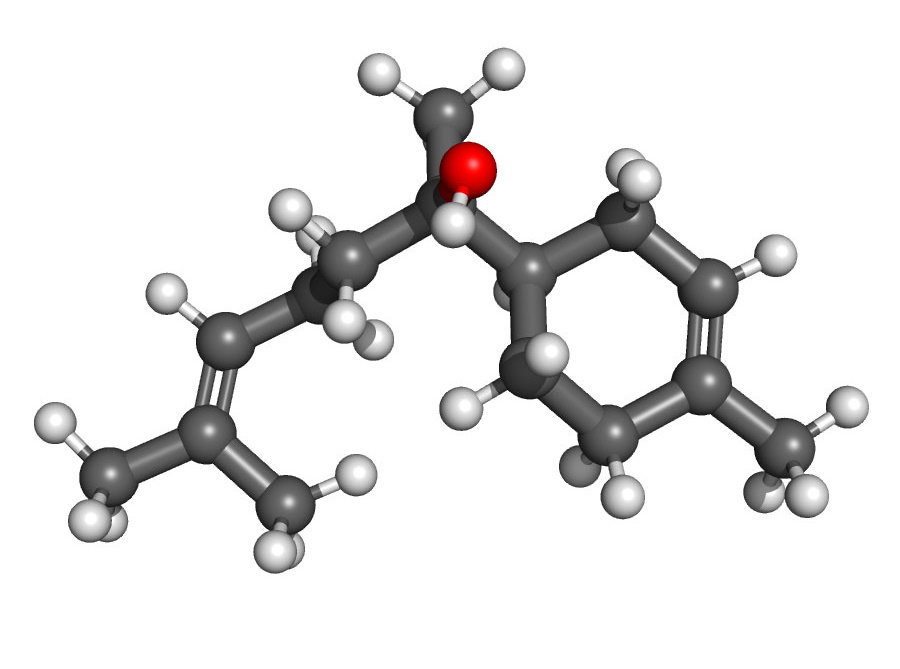
In this case, there is just one oxygen atom, which still earns bisabolol the ‘oid’ designation as a terpenoid. If bisabolol did not feature this one oxygen atom, it would be called a ‘terpene,’ and not a terpenoid.
The addition of one or more oxygen atoms generally changes the functionality of a molecule, but it doesn’t necessarily make it more or less therapeutically valuable. The overall functionality of a molecule depends on the bonding and interactions of the atoms, functional groups, side chains, rings, torsion, and other variables. But when it comes to terpenoids, flavonoids, and cannabinoids, we can immediately say for certain that the compound contains oxygen atoms when we see ‘oid’ in the name. However, there is a bit more to this story when it comes to terpenes.
Terpenes are hydrocarbons ONLY; they consist only of hydrogen and carbon atoms. When this simple small molecule changes to include a functional group like a methyl group or a hydroxyl group, or it gains any atoms that are not hydrogen or carbon, then this is referred to as a terpenoid.